Cardi B said fast food in Colombia tastes "fresh. It was like they cut the chicken in the back." But American fast food tastes "terrible."
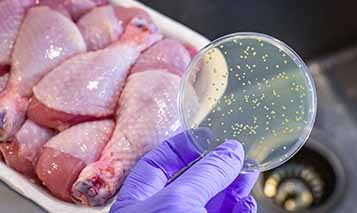
Health officials linked a listeria outbreak to salads and dairy products from Whole Foods and other supermarkets. An FDA inspection linked listeria bacteria.
Americans are dying at an alarmingly high rate since 2019 -- but health experts are "baffled" at the cause
The US. Food & Drug Administration says a decongestant found in Benadryl, Sudafed and DayQuil doesn't work
Rejoice ladies, the U.S. Food & Drug Administration has approved the first over-the-counter birth control pill
The U.S. is moving further away from slaughtering animals for human consumption
Hair straighteners are essential in Black hair salons to tame naturally kinky coils, also known as brittle 4C hair
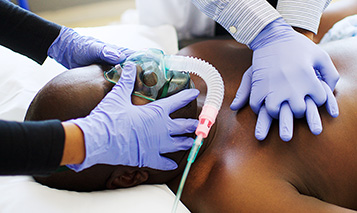
The Food & Drug Administration (FDA) admits the COVID mRNA vaccine is linked to blood clots - but only in the elderly population, age 65 and older
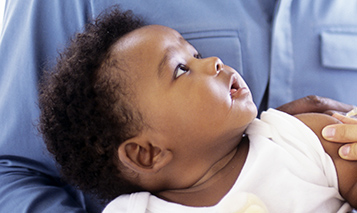
The Food & Drug Administration (FDA) has authorized bivalent Pfizer and Moderna COVID-19 vaccines for babies as young as six months
The Food and Drug Administration has approved a first-of-its-kind treatment for the skin condition vitiligo