The Centers for Disease Control is recommending a fourth Covid-19 mRNA booster shot for people 18 and over who are “moderately and severely immunocompromised”.
The CDC updated its guidelines on Tuesday to add recommendations for people who are immunocompromised and have received 2 doses of mRNA (Pfizer-BioNTech, Moderna or Janssen) plus an additional booster shot.
“Moderately and severely immunocompromised people aged ?18 years who completed an mRNA COVID-19 vaccine primary series and received an additional mRNA vaccine dose may receive a single COVID-19 booster dose (Pfizer-BioNTech, Moderna or Janssen) at least 6 months after completing their third mRNA vaccine dose.
“In such situations, people who are moderately and severely immunocompromised may receive a total of four COVID-19 vaccine doses.”
Also on Tuesday, an FDA advisory panel voted 17-0 to recommend Pfizer mRNA vaccines for children ages 5-11.
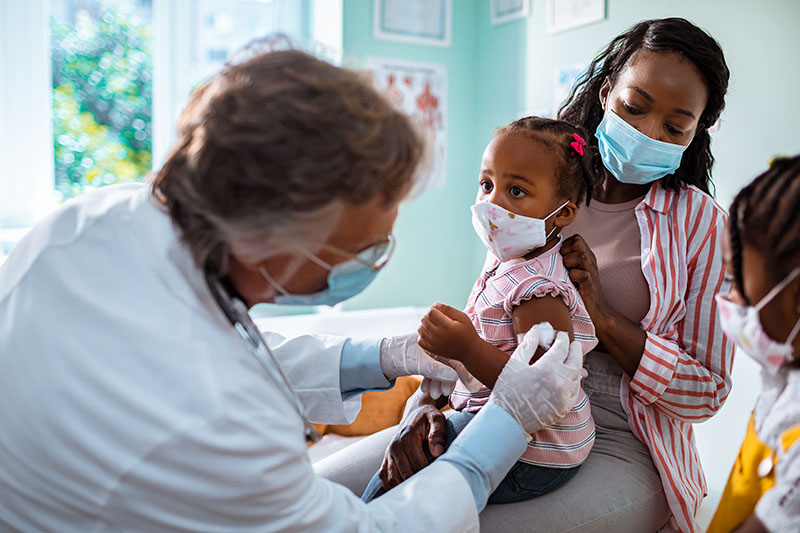
The FDA advisory panel agreed that the benefits of injecting children with mRNA vaccines “outweighs the risks.”
The FDA is not bound by the advisory panel’s recommendation and will make its own final determination within a few days.
A CDC vaccine advisory group will then vote to approve the FDA’s recommendation.
Children as young as 5-11 could begin receiving half of the adult dose of mRNA vaccine next month.