The Food & Drug Administration (FDA) has authorized bivalent Pfizer and Moderna COVID-19 vaccines for babies as young as six months.
The FDA authorization covers Pfizer and Moderna vaccines that target both the original coronavirus and Omicron sub-variants for use in children as young as 6 months through 5 years old.
The amended authorization on Thursday from the FDA authorizes use of Moderna’s bivalent shot as a third booster shot in children 6 months through 5 years of age, two months after their initial vaccination.
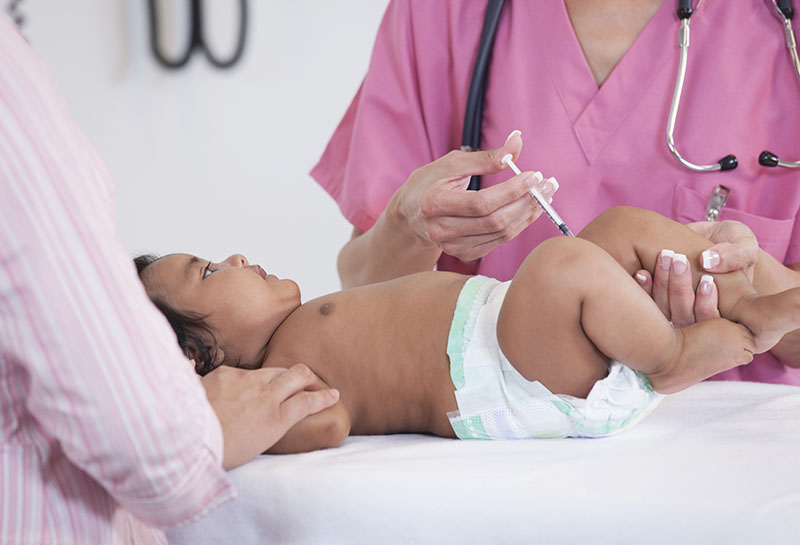
“More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine… especially as we head into the holidays and winter months where more time will be spent indoors,” FDA Commissioner Robert Califf said in a statement.
The Centers for Disease Control (CDC) is urging parents to get their babies vaccinated before the holidays.
The Pfizer vaccine for babies is a 3-shot regimen given over at least 11 weeks.
Overall, 39.7 million people in the U.S. have received a bivalent booster as of Nov. 30, data from the CDC showed.