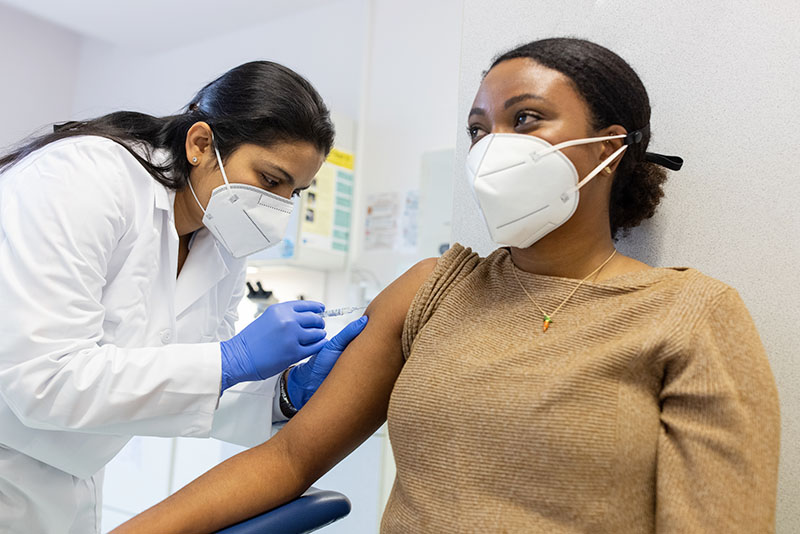
The U.S. Food & Drug Administration has approved the first injectable HIV prevention drug for adults and adolescents.
Apretude is administered one month apart, then every two months to prevent the risk of acquiring HIV.
The long-acting cabotegravir (or Apretude) injectable drug is approved for at-risk adults and adolescents who weigh at least 77 pounds.
Apretude is not intended for people who are already diagnosed with HIV or AIDS.
READ ALSO: CDC recommends HIV drug PrEP for everyone who is sexually active
The news comes about a month after the Centers for Disease Control updated its guidelines recommending physicians talk to all sexually active adult and adolescent patients about taking PrEP (pre-exposure prophylaxis).
“I really think this puts PrEP in the same place as so many other really good preventive interventions like talking about smoking, alcohol, drugs, etc,” Dr. Demetre Daskalakis, the director of CDC’s Division of HIV Prevention, told CNN.
The injectable drug is expected to replace the daily PrEP pill for high-risk individuals such as men who have sex with men (MSM).
“Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill,” said Debra Birnkrant, director of the Division of Antivirals in the FDA’s Center for Drug Evaluation and Research.